One of the most common causes of cancer- related mortality in women is breast cancer [1]. Modified radical mastectomy (MRM) is one of the preferred methods in the surgical treatment of breast cancer. In MRM, the breast tissue, fascia of the pectoral major muscle, and level I and II axillary lymph nodes are removed [2]. This surgery is performed with a wide incision and causes moderate to severe postoperative pain. In addition, postoperative chronic pain has been reported in 25–60% of these patients [3, 4]. Chronic pain has a major negative impact on the quality of life due to the physical limitations and psychological distress it causes. There is also evidence that acute postoperative pain is closely related to the chronic pain experienced in the following months [5]. Therefore, effective postoperative pain management is important not only for the early postoperative period but also for the prevention of chronic pain that will last for months.
Many analgesic protocols are used for pain control after MRM, but none are ideal [6]. For example, intravenous patient-controlled opioid analgesia is commonly used but often associated with nausea/vomiting and excessive sedation [7]. Techniques, such as paravertebral and thoracic epidural blocks significantly reduce postoperative pain scores and opioid consumption, but they are technically difficult to implement and their associated complications, although rare, can be life-threatening [6]. In recent years, ultrasound-guided facial plane blocks have been defined as alternative methods to neuraxial techniques. Pectoral nerve blocks (PECS-I and PECS-II), serratus plane block (SPB), rhomboid intercostal and sub-serratus block (RISS), and erector spinae plane block (ESP) have been shown to be effective in the management of postoperative acute pain in various studies; however, there is only limited available research on their efficacy in chronic pain after breast surgery [8–12].
This primary aim of the presented study was to investigate the influence of ultrasound-guided SPB on postoperative chronic pain at 6 months after surgery in patients undergoing MRM and axillary lymph node dissection (AD). Our secondary objectives in this study were to evaluate postoperative acute pain, opioid consumption, and quality of life of patients.
METHODS
After ethics committee approval of Ataturk University Faculty of Medicine (clinical research assembly number: 6, decision number: 12; date: 24 October 2016), this study was conducted with patients aged 18–65 years, ASA I–III, who were scheduled for selective MRM and AD, did not have known heart disease, kidney disease, liver disease, allergy, or chronic pain, and who agreed to participate in the study. Patients with neurological disease or coagulopathy, those using anticoagulant drugs, those who had not undergone axillary dissection, cases in which surgery was modified after the initial decision, those who could not cooperate, and those with body mass index (BMI) over 35 kg m–2 were excluded from the study.
Patients were randomly assigned to one of two equal groups, including 30 patients each, using a computer-generated randomization table (Microsoft Office 365 Excel [Microsoft, Redmond, WA, USA, http://www.microsoft.com]) and concealed sealed opaque envelopes. An investigator who had not participated in the subjects opened the sealed opaque envelopes containing the group allocation information. The injected drugs were prepared by an anaesthetist who did not participate in this study and was unaware of the patient group allocation. All researchers, surgeons, participants, caregivers, and patient outcome evaluators who recorded the postoperative data were unaware of the group assignment. Patients in the serratus plane block (SPB) group received subcutaneously 30 mL of 0.25% bupivacaine, while patients in the control group received 2 mL of saline placebo (saline).
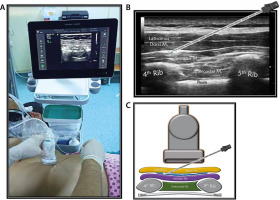
SPB group (n = 30): After being taken to the regional anaesthesia room, the patients were provided standard monitoring and placed in the lateral decubitus position with the area to be treated on top. The area to be injected and the high-frequency linear USG probe were prepared under sterile conditions. The ultrasonography probe was placed on the fourth and fifth ribs of the midaxillary line with a longitudinal parasagittal orientation (Figure 1A). The subcutaneous adipose tissue of the skin, latissimus dorsi muscle, serratus anterior muscle, ribs, and pleura were consecutively visualized. A sonovisible nerve block needle was advanced between the latissimus dorsi and serratus muscles using the in-plane orientation (Figure 1B). After it was observed that there was no blood nor air by aspiration, the needle location was confirmed with 1–2 mL of saline, and 30 mL of 0.25% bupivacaine was applied.
FIGURE 1
a) Patient, ultrasound setup and needle orientation for serratus plane block (SPB). B) Ultrasound anatomy of SPB. C) Basic illustration of SPB
Control group (n = 30): The patients were positioned as described in the SPB group. Sterilized conditions were met, and ultrasound-guided 2 mL of saline was injected subcutaneously.
After the block procedure, routine general anaesthesia was performed in all patients in both groups using propofol (2–3 mg kg–1), fentanyl (1–2 µg kg–1), and rocuronium (0.6 mg kg–1) intravenously. Anaesthesia was maintained with 1–2% sevoflurane and 60% N2O–40% O2. After surgery the patients’ tracheas were extubated and patients taken to the post-aesthetic care unit (PACU). All surgical procedures were performed by the same surgical team using the same technique.
Postoperative analgesia protocol
The same postoperative analgesia protocol was administered for both groups. All patients were given 50 mg dexketoprofen trometamol intravenously 30 minutes before the end of the surgery. The same dose was repeated every 12 hours postoperatively. A patient-controlled analgesia device (PCA) containing fentanyl was administered in the postoperative recovery room. The PCA was set at a 10 μg mL–1 concentration, with a 50 μg loading dose, 10 minutes lockout time, a bolus of 25 μg, and without basal infusion, and it was left connected for a day. Patients with a Visual Analogue Scale (VAS) score of 4 and above in PACU were administered 25 mg meperidine and noted. A blinded researcher undertook the postoperative follow-up of the cases.
Evaluation of postoperative acute pain
Post-surgical pain assessment was performed by VAS based on a scale of 0 to 10 (0 = no pain, 10 = the most unbearable pain ever experienced). The VAS scores at hours 1, 2, 4, 8, 12, and 24 were recorded in PACU. In addition, the amount of fentanyl intake was recorded for the intervals of 0–4, 4–8, and 8–24 hours, and the total intake was obtained for the 24-hour period. The time to first analgesic requirement was defined and recorded as the time when the VAS score was ≥ 4. In addition, postoperative opioid-related side effects, such as hypotension, vomiting, and nausea were noted.
Evaluation of chronic pain and quality of life
After surgery, persistent pain was evaluated using a numerical rating scale (NRS) (0: no pain, 10: worst pain imaginable) at the postoperative first and sixth months. The painDETECT questionnaire (PDQ) characteristics were assessed, consisting of 9 items, without requirement of an additional point-of-care clinical examination. A multi-centre study showed that PDQ had 85% sensitivity, 80% specificity, and 83% accuracy in classifying pain as neuropathic and nociceptive [13]. The PDQ has been officially validated in a Turkish population, which was also used in the current study [14]. A score between 0 and 38 is calculated from 9 questions in PDQ, with higher scores indicating a higher likelihood of neuropathic pain. A PDQ score of 0–12 indicates nociceptive pain (probability of a neuropathic pain component < 15%), a score between 13 and 18 is uncertain (an indeterminate neuropathic pain component may be present), and a score between 19 and 38 indicates possible neuropathic pain (> 90%).
The quality of life and general health status of the patients were evaluated using the Short Form-36 (SF-36) at the postoperative first and sixth months. The SF-36 is a questionnaire with 8 subscales, which provides information about a person’s health status and quality of life. The SF-36 was translated into Turkish in 1999. Validation studies have been undertaken in different patient groups [15–17].
Sample size determination and statistical analysis
We determined that we would need 25 patients in each group to have an 85% power to detect a reduction in the rate of chronic pain from 40% to 20%, with 2-sided test at an a probability of 0.05 based on previous studies [18]. We scheduled to enrol 60 participants to account for possible dropouts.
SPSS version 20.0 (SPSS Inc., Chicago, Illinois, USA) program was used for the analysis. The normal distribution of data were checked using a histogram and the Kolmogorov-Smirnov Z test. The independent samples t-test was used for normally distributed data, and the Mann-Whitney U test was used for non-normality distributed data. For categorical variables, the c2 test was used. Intra-group evaluations were undertaken with the paired samples t-test. P-value < 0.05 was considered statistically significant.
RESULTS
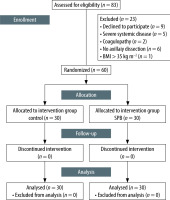
Eligible patients for the study were analysed for the primary outcomes. The flow diagram of the study is presented in Figure 2. The demographic data of groups are shown in Table 1. There was no statistically significant difference between the groups in terms of age, weight, height, BMI, ASA classification, duration of surgery, duration of anaesthesia, length of incision, and presence of chemoradiotherapy (P > 0.05) (Table 1).
TABLE 1
Demographic and clinical data of study
When the postoperative VAS scores of the control and SPB groups were compared, it was determined that these scores were statistically significantly higher in the former than in the latter; measurements were taken at PACU at the 1st, 2nd (P < 0.001), and 4th hour (P = 0.014). However, there was no statistically significant difference for the VAS scores obtained at hours 8, 12, and 24 (P > 0.05) (Table 2).
TABLE 2
Acute postoperative pain scores
Postoperative fentanyl consumption was evaluated at the intervals of 0–4 hours, 4–8 hours, and 8–24 hours, and the total consumption was measured at 24 hours. Fentanyl consumption was statistically significantly lower in the SPB group than in the control group at all evaluation times (P < 0.001). Also, additional analgesic was required in 10 patients in the control group but only in 3 patients in the SPB group, indicating a statistically significant difference (P = 0.028) (Table 3).
TABLE 3
Opioid consumption time intervals and rescue analgesia
In the assessment of persistent postoperative pain, 23% of the patients in the control group and 20% of those in the SPB group had moderate to severe pain (VAS ≥ 4) at the first month (P = 0.754) while moderate to severe pain was determined in 16% and 6% of the patients, respectively, at the sixth month (P = 0.228) (Table 4).
TABLE 4
Persistent pain evaluation
The neuropathic pain characteristics of the patients were evaluated with PDQ. At the first month, 10% of the patients in both groups showed a high probability of a neuropathic pain component. At this evaluation time, the mean PDQ score was 9 (3–21) for the control group and 8.5 (2–20) for the SPB group, with no statistically significant difference between the groups (P = 0.764). When PDQ was evaluated at the sixth month, 10% of the patients in both groups showed a high probability of a neuropathic pain component, with the mean PDQ score being determined as 8 (0–22) and 7.0 (0–22) for the control and SPB groups, respectively (P = 0.684) (Table 5).
TABLE 5
PainDETECT scores and neuropathy classification of the study groups
The general health status and quality of life of the patients were evaluated using SF-36 at the first and sixth months. Accordingly, there was no statistically significant difference between the 2 groups at the first and sixth months in terms of the SF-36 parameters (P > 0.05) (Table 6).
TABLE 6
Short Form-36 scores of the study groups
DISCUSSION
This study showed that ultrasound-guided SPB reduced the acute postoperative pain scores, 24-hour opioid consumption, and the need for rescue analgesia compared to the control group in the patients undergoing MRM and AD. On the other hand, the incidence of chronic pain was low and not significantly affected by the pre-emptive SPB.
The innervation of the breast tissue and the axillary region is provided by nerves originating from different regions. While lateral and median pectoral nerves originating from the brachial plexus are responsible for most of the innervation of the pectoralis major and minor muscles, the intercostobrachial, thoracodorsal, and long thoracic nerves play a role in the axillary area. The second and main group of nerves that provide the innervation of the breast tissue are the anterior divisions of the T2–T6 intercostal nerves. The intercostal nerves extend between the internal intercostal muscle and innermost intercostal muscles to the sternum. The lateral cutaneous nerve branch emerges from the midaxillary line, and the pectoral region provides skin innervation up to the parasternal area. The sensation of the parasternal region is supplied by the anterior cutaneous nerves branching from the intercostal nerves [19–21].
SPB is performed at the fourth-fifth rib level at the midaxillary line by injecting local aesthetic along the superficial or deep fascia of serratus anterior muscle, referred to as superficial and deep SPB, respectively. SPB blocks the second to sixth intercostal nerves, as well as the thoracodorsal and long thoracic nerves, and involves the use of anaesthesia in a wide area that includes the T2–T9 dermatome areas and the axillary region in the lateral, anterior, and posterior parts of the thoracic wall. In a study conducted with voluntary patients, Blanco et al. [22] showed that almost complete anaesthesia was achieved in the hemithorax where SPB was applied, and superficial SPB (750–840 min) had a longer effect than deep SPB (330–600 min). We also preferred superficial SPB in our study. While the postoperative VAS pain scores were lower in the patients who underwent the SPB procedures compared to the control group, especially for the first four hours, the mean VAS pain scores were below 4 for all patients at hour 24.
SPB is widely used for postoperative analgesia in breast and thoracic surgery. In a systematic review and meta-analysis examining a total of 1260 patients who underwent 6 thoracic surgical operations and 13 breast operations involving the SPB procedure, Chong et al. [23] showed that SPB reduced the postoperative pain scores, prolonged the time to first analgesic requirement, and prevented nausea and vomiting by reducing the postoperative opioid need. In our study, in line with the literature, opioid consumption at all evaluation times and the total opioid requirement were lower in the patients who underwent SPB.
Acute pain is a complex physiological and psychological response to tissue trauma and associated inflammatory processes. This pain arises in response to tissue damage and inflammation and is normally limited to a certain period of time. However, if postoperative acute pain and accompanying physiological processes are not adequately controlled, pain may become chronic [24]. Chronic pain development after breast surgery is multifactorial, and its pathogenesis remains unclear. The psychosocial status of the patient depends on various factors, such as age, obesity, ethnicity, genetics, sensory disorders, lymph node dissection, lymphoedema developing in the chronic period, adjuvant chemo-radiotherapies, and malignancy recurrence. It is also closely related to complications associated with surgery, and in most cases, chronic pain has been attributed to nerve damage. In mastectomy and axillary lymph node dissection, the intercostobrachial nerve and thoracic intercostal nerves can be damaged [25]. During surgery, direct damage to the nerves, such as rupture, compression, ischaemia, stretching, and retraction may develop, or nerve damage may occur later due to traumatic neuroma or scar tissue [26–28].
The main treatment strategy for postoperative chronic pain is to reduce peripheral and central neuronal sensitization and suppress inflammatory responses [29]. Therefore, regional anaesthetic methods are used as a part of multimodal analgesia to prevent and treat chronic postoperative pain, but their efficacy is controversial. In a retrospective study conducted with patients who had undergone an elective inguinal hernia operation, Paasch et al. [30] found that the transversus abdominis plane (TAP) block applied for postoperative analgesia could reduce postoperative chronic pain. In contrast, in a prospective study, although the TAP block significantly reduced acute pain, the incidence of chronic pain was not significantly affected [31].
Qian et al. [32], comparing the preoperative multilevel paravertebral block with a control group in terms of chronic postmastectomy pain, reported that chronic pain was lower in the paravertebral block group at the postoperative third month (34.5% vs. 51.2%) and sixth month (22.1% vs. 37.2%) compared to the control group. However, studies evaluating the effectiveness of facial plane blocks in chronic pain in breast surgery are limited. Although there is no research in the literature comparing SPB with a control group for chronic pain, one study compared SPB with the PECS-II block. In that study, Fujii et al. [33] showed that both blocks were effective in the management of postoperative acute pain. In addition, although the health-related quality of life was similar in the patients who underwent SPB and the PECS-II block at the postope-rative sixth month, the rate of moderate-severe pain was found to be lower in the latter (10%) compared to the former (33%). In our study, at the end of the sixth month, we detected an NRS score of ≥ 4 in 11% of all patients, and a PDQ score of 19 and above in 10% of all patients. Furthermore, moderate to severe chronic pain developed in 2 patients in the SPB group and 5 patients in the control group, indicating no significant difference.
In the current study, intravenous dexketoprofen was given to all patients at the end of the surgery, and PCA was applied with fentanyl for 24 hours postoperatively. In addition, postoperative acute pain was well managed in both groups by applying rescue meperidine when necessary. It is known that high pain levels in the early postoperative period are closely related to chronic pain [34]. We consider that the low incidence of chronic pain in our study is closely related to the effective management of postoperative acute pain in both groups.
It is important to investigate preventable factors that play a role in the aetiopathogenesis of chronic pain, in order to increase the quality of life of these patients. The most important of these factors is to reduce surgical complications, especially nerve damage. It is predicted that the risk of nerve damage and chronic pain can also be decreased depending on surgical expertise [35]. It is known that neuralgia due to intercostobrachial nerve injury plays a major role in chronic pain in breast cancer surgery [28]. In our study, we expected less neuropathic pain in the SPB group because this procedure blocked the intercostobrachial nerve, but the number of patients that had a PDQ score of 19 and above, and thus were accepted as positive for neuropathic pain, was equal for both the control and SPB groups. The surgical team that performed all operations in our study has been performing breast surgery for more than 20 years and is specialized in this field. Therefore, we consider that the low incidence of neuropathic pain in both groups may be related to the experience of the surgical team.
This study has some limitations. First, the preoperative pain scores of the patients due to malignancy were not evaluated. Preoperative pain status may affect postoperative pain scores and opioid consumption. Second, the psychosocial status of our patients was not assessed before the operation. Third, the chemo-radiotherapy protocols could not be fully analysed, and the malignancy recurrence was unknown. Fourth, this study has not been prospectively registered on a database. Lastly, although lymph node dissection was performed in all of our patients, we did not separately evaluate arm-shoulder pain.
CONCLUSIONS
We have shown that ultrasound-guided SPB demonstrated superiority versus the control group with respect to acute postoperative pain parameters as pain scores, opioid consumption, and rescue analgesia. On the other hand, the incidence of chronic pain was low and not significantly affected by the pre-emptive SPB in the patients undergoing MRM and AD. More research is needed with larger sample size and longer follow-up intervals.