Malnutrition in surgical patients remains a common medical issue negatively affecting the perioperative period. Assessment of nutritional status should belong to routine physical examinations in every patient. One of the tools used to diagnose malnutrition is the Global Leadership in Malnutrition (GLIM) criteria [1]. The GLIM criteria assess both aetiological and phenotypical criteria to determine malnutrition. Unlike other nutritional assessment protocols, GLIM is not a screening tool but a diagnostic one. Almost 20% of elective surgery patients meet the GLIM criteria for malnutrition, and this prevalence rises to 40% in patients scheduled for elective surgery for cancer [2]. Oesophageal and oesophago-gastric junction cancer is a disease associated with one of the highest malnutrition rates among all surgical cancer patients, reaching up to 80% [3]. Major causes of malnutrition in this population include dysphagia, nausea, and loss of appetite, as well as frequently performed preoperative chemoradiotherapy, which further reduces body weight and food intake. Identifying malnourished patients with oesophageal cancer is one of the important factors regarding the potential improvement of the perioperative period.
Given that malnutrition negatively affects surgical outcomes, increases complication rates and mortality, prolongs hospital stays and increases costs, proper identification of patients at risk of malnutrition should be a priority in medical care [4]. Preoperative nutritional interventions benefit patients, reduce the complication rates and improve overall outcomes [5]. Monitoring preoperative nutritional interventions remains a challenge for dieticians and clinicians, as it involves finding the optimal moment for surgical intervention with all the benefits of preoperative nutrition. Exploring the impacts of nutritional interventions on the perioperative period and finding new markers for determining appropriate nutrition are topics of interest among clinicians. One postulated malnutrition marker is total lymphocyte count, which correlates with poor nutritional status in surgical patients [6]. However, despite the reduced total lymphocyte count in malnourished patients, we often observe an increased total white blood count in patients with malnutrition risk, suggesting that other white blood cells might also be affected by patient nutritional status [7, unpublished data]. Since malnutrition often promotes chronic inflammation, detecting chronically activated immune cells could help determine metabolically malnourished patients. Among potential new parameters of chronic inflammation are those related to neutrophil activity, i.e., neutrophil reactivity intensity (NEUT-RI) and neutrophil granularity intensity (NEUT-GI), which are indicative of the activation stage of the neutrophilic granulocytes. This measurement, available in the Sysmex Diagnostic haematology analysers, considers the metabolic activity of neutrophils, the internal structure, and the size of the cell [8]. The usefulness of NEUT-RI and NEUT-GI as markers of systemic inflammation has previously been demonstrated in autoimmune hepatitis patients compared to healthy volunteers [8]. The usefulness of NEUT-RI and NEUT-GI as markers of malnutrition and chronic inflammation in elective surgical patients is yet to be determined.
Thus, the primary aim of this study was to establish the correlation between NEUT-RI and NEUT-GI and nutritional status of patients scheduled for elective oesophagectomy due to cancer.
METHODS
This was a prospective observational study approved by the local bioethics committee (KE-0254/ 142/2020). This study was performed in adherence to the Declaration of Helsinki and Good Clinical Practice guidelines. Before enrolling in the study, each patient signed an informed consent form.
The primary aim of this study was to establish the correlation between NEUT-RI and NEUT-GI and nutritional status in patients scheduled for elective oesophagectomy due to cancer.
Secondary aims of this study were to determine the factors associated with the mortality of elective oesophageal cancer patients and to assess the impact of nutritional responsiveness on the perioperative period.
We recruited patients scheduled for elective oesophagectomy due to adenocarcinoma (AC) or squamous cell carcinoma (SCC) of the oesophagus or oesophago-gastric junction between 2021 and 2023.
Inclusion criteria included patient’s age between 18 and 80, scheduled for radical double-field resection of the tumour.
We excluded patients receiving steroids or immunosuppression, unable to consume nutrition orally, undergoing palliative surgery due to an unresectable tumour, treated for chronic inflammatory diseases (i.e. asthma, chronic obstructive pulmonary disease) or autoimmune disease (i.e. thyroiditis, type 1 diabetes, inflammatory bowel diseases, autoimmune hepatitis), diagnosed with other than oesophageal malignancies, unable to provide reliable information about the preoperative period – especially about body weight monitoring and nutritional interventions.
Detailed medical histories were obtained before surgery, including patient demographics: age, height, weight, body mass index (BMI), type of cancer, preoperative chemoradiotherapy, reported maximum preoperative body weight loss, regain of body weight before surgery, use of oral nutritional support (ONS) before surgery, duration of ONS use, adherence to ONS use, other nutritional interventions (e.g. enteral or parenteral nutrition), chronic diseases, GLIM status and preoperative fasting time. Patients’ laboratory results before surgery were recorded, including blood morphology, neutrophil-to-lymphocyte ratio (NLR), NEUT-RI, NEUT-GI, C-reactive protein, serum albumins, serum proteins, serum ketone bodies, renal parameters, electrolytes. Post-surgical data included routine laboratory test results (total blood count, electrolytes, C-reactive protein, procalcitonin, renal parameters), duration of intensive care unit (ICU) stay in days, cost of ICU stay in euro (we used a conversion rate of four and half polish zloty to one euro), duration of in-hospital stay, complications based on the Clavien-Dindo classification [9], and ICU and in-hospital mortality.
We obtained information from the patients concerning their preoperative period, pre-disease weight, and body weight loss after they were sche-duled for the surgery. Thus, our interviews were biased by patients’ recalls and self-assessments.
Preoperative use of ONS was considered fulfilled if it lasted for at least seven consecutive days and with the dosing recommended by the manufacturer, as recommended by the European Society for Clinical Nutrition and Metabolism for surgical patients [10]. Patients had freedom to choose ONS themselves, and all patients who used ONS reported using either Nutridrink Protein, Nutridrink Protein Omega-3 (Nutricia, Poland) or Fresubin Protein Energy (Fresenius Kabi, Poland) two to three times daily. Primary care physicians, oncologists, and surgeons recommended using ONS in our patients. If necessary, we modified the ONS consumption regime in the preanesthetic clinic at least 14 days before a scheduled operation.
Patients were divided into two groups: nutritional responders (R group) and non-responders (NR group). Nutritional responsiveness was a novel concept created for the needs of this study and was defined as regaining at least 25% of the maximum preoperative body weight loss before the surgery with the introduction of nutritional intervention (including ONS and/or increased food intake). This concept was created due to a lack of data in the lite-rature about measurable goals of nutritional intervention, especially in prolonged cases as in oeso-phageal cancer patients.
Blood samples for NEUT-RI and NEUT-GI were obtained the day before surgery during routine presurgical blood drawing. The analysis was performed within one hour of blood drawing using the Sysmex XN 1500 apparatus (Sysmex Europe SE, Warsaw, Poland). NEUT-RI is expressed in FI units, describing the fluorescence intensity, whereas NEUT-GI is expressed in SI units, describing the light intensity of the scattered laser beam.
Blood samples for serum ketone analysis were obtained on the morning of the day of surgery. We used two methods to determine ketone serum levels. The bedside B-hydroxybutyrate was assessed using the Optium Xido Neo (Abbott, Illinois, USA).
Additionally, for chromatographic assessment, a 2 mL blood sample was centrifuged within 15 min of collection at 3,000 rpm for 10 min. The sample tube with the serum was then immediately cryopreserved in a −80-degree Celsius freezer and stored there until transportation to the laboratory. Acetone determination was performed using the GC/FID-headspace two-column method on a gas chromatograph Trace GC Ultra (2 FID detectors and split/splitless injector) coupled with a TriPlus HS autosampler (Thermo Fisher Scientific, Waltham, MA, USA).
The detailed methodology of the ketone body assessment was presented in our previous study [11].
We collected statistical data in a Microsoft Excel (Microsoft, Redmond, WA, USA) spreadsheet. We present categorical variables as numbers and frequencies and analyse them using Fisher’s exact test. Continuous variables were tested for normal distribution using the Kolmogorov-Smirnov test, Lilliefors test, and the Shapiro-Wilk test. Normally distributed continuous variables are presented as means and standard deviations of the mean and analysed using Student’s t-test. Non-normally distributed variables are presented as medians and interquartile ranges (IQRs) and analysed using the Mann-Whitney U test. We performed all statistical calculations using Statistica 13.3 (StatSoft Inc., Tulsa, OK, USA).
RESULTS
Demographics
We included in this study 25 patients, 21 of whom were male (84%). The mean age was 56.4 years, the mean BMI was 23.6 kg m–2, the mean reported percentage body weight loss before nutritional intervention was 17.6% of the patient’s body weight before the disease, and 96% (24/25) of patients met the GLIM malnutrition criteria at some point within six months before surgery. Of all the patients who met the GLIM criteria, 16.7% (4/24) presented with moderate malnutrition, and 83.3% (20/24) presented with severe malnutrition, at some point in the preoperative period. On the day of surgery, 80% (20/25) of patients met the GLIM criteria, 30% (6/20) presented with moderate malnutrition, and 70% (14/20) continued to be classified as severely malnourished. The mean preoperative fasting time was 10 hours. The type of cancer was 48% (12/25) AC and 52% (13/25) SCC. The distribution of patients based on the T grade was T1 – 12% (3/25), T2 – 32% (8/25), T3 – 44% (11/25), (3/11) T4 – 12% (3/25).
Mortality, complications, ICU and in-hospital duration and costs
Overall, in-hospital mortality was 28% (7/25). Mortality based on the T grade was as follows: T1 – 0%, T2 – 12.5% (1/8), T3 – 27.3% (3/11), T4 – 66% (2/3). Apart from the T grade, the only preoperative factor associated with mortality was the presence of nutritional responsiveness: 11.1% vs. 71.4% (P = 0.008). The use of ONS (21.1% vs. 50%; P = 0.17) or type of cancer (8.3% AC vs. 46.2% SCC; P = 0.072) did not significantly affect mortality. No recorded preoperative laboratory results were found to be associated with an increased risk of mortality.
The most common complication was refeeding syndrome, which was present in 88% (22/25) of all patients. Based on the Clavien–Dindo classification, 32% (8/25) of patients developed grade I complications due to electrolyte disturbances. Grade III was observed in 32% (8/25), mostly endoscopic prothesis and pleural drainage. Grade IV developed in 8% (2/25) of patients, who required renal replacement therapy and prolonged mechanical ventilation. Grade V presented in 28% (7/25) of patients, who died during their hospital stay; the main cause was mediastinitis.
The median ICU stay was seven days, and the median in-hospital stay was 17 days. Patients with AC had significantly shorter in-hospital stays than SCC patients: 13 (9–20) vs. 32 (15–37) days, respectively (P = 0.02). The ICU stay difference between types of cancer was not statistically significant: 5.5 (4–7.5) days for AC vs. 11 (6–26) days for SCC (P = 0.07). The R group had significantly shorter ICU stays: 5.5 (4–8) vs. 13 (7–31) days (P = 0.01). Shorter ICU stays resulted in a lower cost of ICU stays in the R group: 4775.2 (3938.9–7640.7) vs. 12255.8 (7787.6–49108.7) euro in the NR group (P = 0.01).
Nutritional responders vs. non-responders
In our study, we observed 18 nutritional responders (R group) and seven non-responders (NR group). The characteristics of both groups are presented in Table 1.
TABLE 1
The data for the preoperative period for the R group and NR group. The data are presented as the number of patients and percentages for categorical data and are calculated using the χ2 test, means and standard deviations for normally distributed continuous data are calculated using the t-test, and medians and interquartile ranges for non-normally distributed continuous data are calculated using the Mann-Whitney U test
Between the R group and the NR group, we observed statistically significant differences in both NEUT-RI and NEUT-GI values (see Figures 1 and 2).
FIGURE 1
The difference in NEUT-RI values between the R group and NR group
R group – nutritional responders, NR – nutritional non-responders
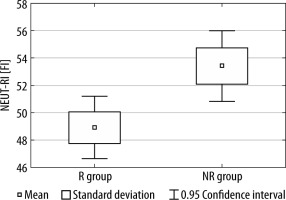
FIGURE 2
The difference in NEUT-GI values between the R group and NR group
R group – nutritional responders, NR – non-responders
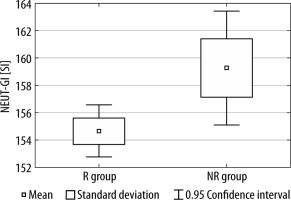
There was no statistically significant difference between NEUT-RI and NEUT-GI in terms of mortality (NEUT-RI, P = 0.35; NEUT-GI, P = 0.65), type of cancer (NEUT-RI, P = 0.23; NEUT-GI, P = 0.25), preoperative use of ONS (NEUT-RI, P = 0.52; NEUT-GI, P = 0.47) or preoperative chemoradiotherapy (NEUT-RI, P = 0.61; NEUT-GI, P = 0.40).
Ketone bodies
We found no statistically significant difference in ketone serum levels measured using bedside devices: mortality (P = 0.74), type of cancer (P = 0.41), preoperative use of ONS (P = 0.88), nutritional responsiveness (P = 1.0) and preoperative chemoradiotherapy (P = 0.44).
The median serum acetone was 0.06 (0.04–0.21) in the R group and 0.16 (0.03–0.42) in the NR group (P = 0.41). No other assessed factor was found to be associated with changes in acetone serum levels: mortality (P = 0.28), type of cancer (P = 0.54) and preoperative use of ONS (P = 0.71). No correlation was found between the percentage of preoperative body weight loss and acetone serum levels (P = 0.56)
DISCUSSION
The results show that the novel neutrophil parameters NEUT-RI and NEUT-GI detected patients who did not respond to nutritional intervention. In this study nutritional responders had a higher chance of survival and generated lower costs in the ICU.
In our study, patients with SCC had a statistically non-significant higher mortality rate than those with AC. Gockel et al. also reported the influence of SCC on poorer nutritional status [12]. In our study, nutritional responsiveness was observed in all AC patients and almost half of the SCC patients, and it was the only preoperative factor associated with significantly reduced in-hospital mortality (71.4% vs. 11.1%). Given that SCC also affected preoperative nutritional status and response to nutritional interventions, underlying causes of malnutrition among different histological type of cancers should be further evaluated. Another factor that significantly improved nutritional responsiveness was preoperative use of ONS; however, use of ONS was not significantly associated with reduced mortality (21.1% vs. 50%). In the case of oesophageal cancer patients, introducing preoperative ONS can improve nutritional responsiveness; however, especially among SCC patients, monitoring of nutritional interventions, as well as body weight gain evaluations, should be performed to ensure the positive impact of the intervention on the perioperative period.
Nutritional responsiveness was also an important factor affecting in-hospital costs. Based on our internal protocols, patients after oesophagectomy are transferred to the ICU, which functions as a post-surgical ward in extended procedures. Nutritional responders had significantly shorter ICU stay durations than non-responders. Stay duration also impacts ICU costs, with more than double the median cost for non-responders. The most expensive non-responder case (49108,7 euro) could have covered the ICU costs of 10 nutritional responders (4775,2 euro). The impact of preadmission malnutrition on hospital costs is well established in the literature, as it increases costs by 34–55% [13]. When the increase in cost affects the most expensive wards in the hospital, we observe a high cash flow that could be used to finance preoperative nutrition and improve patient prognosis. Although the SCC patients had statistically significantly longer in-hospital stays, due to institutional limitations, we only had access to ICU costs.
In our study, we assessed the correlation between laboratory tests and nutritional responsiveness. The only parameters that differed between the R group and the NR group were NEUT-RI and NEUT-GI. These parameters were not affected by cancer type or use of ONS and were not associated with mortality, preoperative chemoradiotherapy or any other factor measured in this study. They defined the reactivity and granularity indexes of the insides of neutrophils, and they differed between the two groups, while the overall number of neutrophils remained similar. The nutritional non-responders had higher values of both NEUT-RI and NEUT-GI, which can be explained by the chronic inflammation observed in malnourished patients [14].
The concept of a strong correlation between inflammation and malnutrition is of interest to many scientists. Using chronic inflammation parameters might provide valuable insight and prognostic value, especially in the case of elective patients, among whom acute inflammation is not present. Chronic inflammation has been identified as a major factor in malnutrition among patients; however, establishing whether malnutrition affects inflammation or vice versa remains elusive [15]. This study did not evaluate whether NEUT-RI and NEUT-GI improved in the R group over the course of the nutritional intervention or whether the R group had lower values from the start, and lowered inflammation status allowed that group to benefit more from nutritional intervention. This remains a goal for future research.
Obtaining laboratory results that could provide additional information on the impact of nutritional therapy remains a research goal of many scientists, especially because markers such as albumins are not very specific and have rather long potency reaching a dozen days. However, the lifespan of neutrophils is about 5.4 days, which might allow for faster observation of nutritional change if a correlation with nutritional interventions can be established [16]. Both hypotheses require further research, as obtaining potential nutritional information from routine blood analysers could improve patient outcomes.
In this study, the variance of ketone bodies with patient nutritional status or other factors assessed in this relatively small population was not statistically significant. In our previous study, we found increased ketone body serum levels in patients with reported preoperative body weight loss; however, in the current study, 96% of patients reported preoperative body weight loss, which may have impacted the results [11]. Additionally, we did not observe any correlation between the severity of preoperative body weight loss and acetone levels. Compared to the previous study, preoperative fasting was much shorter (11 hours in this study vs. 19.75 hours in the previous one), which, considering the nature of ketone body production, may have played a major role in this study’s results. It remains unclear whether the use of ketone bodies as nutritional markers could be feasible, so additional testing in a larger population is required, especially when prolonged preoperative fasting is implemented. While the results of the previous study seemed promising for use of ketone bodies as a nutrition marker, there might be more factors affecting the ketone body serum levels that need to be identified before such application.
This study has some limitations. First, it was a small prospective observational study. The study population, due to the limited number of cases, included both histological types of cancers, which may have affected the results due to different cancers morphologies and pathogenesis that we are not aware of. Second, although we obtained nutritional information from the patients, due to the nature of the study, we could not monitor daily nutritional intake or type of diets that patients consumed in the preoperative period, which may have affected nutritional responsiveness. Finally, since both NEUT-RI and NEUT-GI are relatively novel parameters, we could not rule out the possibility that other factors of which we were unaware affected those parameters.
CONCLUSIONS
Preoperative nutritional interventions are important approaches to improving the oesophagectomy outcome and reduce the ICU costs. Nutritional interventions should be implemented and monitored, as patients who respond to nutritional interventions seem to benefit from them the most. Since proper nutritional status may correlate with neutrophil parameters intensity, future studies should investigate this phenomenon.




