Endoscopic submucosal dissection (ESD) is an ad-vanced endoscopic procedure for treating early-stage gastrointestinal tumours [1]. It enables en-bloc resection of lesions with organ preservation and is associated with better outcomes than other endoscopic resection procedures [2]. ESD requires extensive instrumentation in the gastrointestinal tract, and potential adverse outcomes of gastrointestinal bleeding and perforation have been reported [3, 4]. ESD is generally performed under sedation [5, 6]. However, the use of general anaesthesia (GA) in ESD has also been proposed [7]. GA, when compared with sedation, can minimise inadvertent patient movement [8], which may subsequently improve procedural success rates and reduce complications. We performed a systematic review and meta-analysis to compare GA versus sedation in ESD. The primary objectives of this meta-analysis were to compare the en-bloc resection rates and procedural times when comparing GA against sedation in ESD. We hypothesised that GA would lead to higher en-bloc resection rates without impacting the overall procedural time. The secondary objective of this meta-analysis was to compare the complication rates in GA versus sedation in ESD. We hypothesise that the incidence of intra-procedural hemodynamic instability, gastrointestinal perforation, gastrointestinal bleeding, and aspiration pneumonia would be lower in the GA group than in the sedation group.
METHODS
Protocol
This study was registered in PROSPERO (CRD- 42021275813) and was performed as per the PRISMA guidelines.
Literature search and data extraction
We performed a systematic literature search of EMBASE, MEDLINE and Cochrane Library from inception to 31 August 2021. Search terms (including synonyms or MeSH terms) included a combination of 1) general anaesthesia, 2) sedation, and 3) endoscopic submucosal dissection.
Articles in English comparing GA versus sedation in ESD were included. Papers were not excluded based on year or place of publication. Randomised controlled trials, cohort studies and case-control studies were included. Non-original articles, review papers and conference papers were excluded.
The articles were independently screened by two reviewers (RWHH and CML) for inclusion. Data were subsequently extracted from the included articles independently. Extracted data included patient demographics, indication for ESD, procedural time, rate of en-bloc resection, the incidence of intra- procedural vital instability, the incidence of gastrointestinal perforation, the incidence of gastrointestinal bleeding, and the incidence of post-procedural aspiration pneumonia.
Risk of bias evaluation
The risk of bias was measured by the Cochrane Risk of Bias in Non-randomised Studies of Interventions (ROBINS-I) assessment tool [9], which rated bias in the seven domains of confounding, participant selection, classification of interventions, deviations from intended intervention, missing data, outcome measurement and selective reporting. A final assessment of the overall risk of bias was included. The risk of bias was categorised as low, moderate, high, to critical in each domain.
Level of evidence
The overall evidence level was determined by the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach [10], which assessed parameters including indirectness, potential bias, inconsistency, imprecision and other considerations.
Statistical analysis
Analysis was performed on Review Manager, Version 5.4 (Cochrane Collaboration, Oxford, UK) and MetaXL (Epigear). For articles that reported outcomes in median and range only, mean and standard deviation were calculated through validated methods [11]. Outcomes were compared between patients who received ESD under GA or sedation. The weighted mean difference with 95% CI or relative risk (RR) with 95% CI was calculated for continuous outcomes or dichotomous outcomes, respectively. Subgroup analysis was performed based on the ESD subtype (gastric or oeso-phageal). A random effects model was used for meta-analysis. Qualitative comparison (without statistical analysis) was performed for outcomes that were reported in fewer than three studies.
RESULTS
Study selection
A total of 176 articles were retrieved from the lite-rature search. 169 articles were excluded, and seven articles were selected for analysis. Figure 1 depicts the study inclusion and exclusion process.
Characteristics of included studies
Seven articles involving seven independent studies were included in the meta-analysis [12–18]. The studies were published between 2013 and 2021. Six studies were retrospective cohort studies [12–17], and one study was a prospective cohort study [18]. The studies included a total of 1013 patients (518 receiving GA and 495 receiving sedation). Four studies reported ESD in patients with oesophageal neoplasms [13–16], and two studies reported ESD in patients with gastric neoplasms [12, 17]. One study involved 39 patients with oeso-phageal neoplasms and 66 patients with gastric neoplasms [18]. Table 1 summarises the characteristics of included studies.
TABLE 1
Characteristics of selected studies
Primary outcomes
En-bloc resection
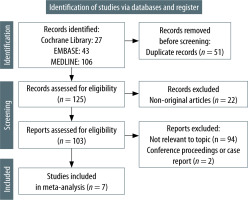
The rates of en-bloc resection were reported in six studies [12–14, 16–18]. The rate of en-bloc resection was 1.04 in GA compared with sedation, although this difference was not statistically significant (RR 1.04; 95% CI: 0.99–1.09; I2 = 71%; P = 0.08) (Figure 2A).
FIGURE 2
Relative risks of en-bloc resection between general anaesthesia and sedation groups in the whole cohort (A) and in oesophageal endoscopic submucosal dissection only (B)
When stratified by ESD subtype, the en-bloc resection rate was higher in GA than in sedation for oesophageal ESD (RR 1.05; 95% CI: 1.00–1.10; I2 = 65%; P = 0.05) (Figure 2B) [13, 14, 16].
No quantitative analysis was performed in the gastric ESD subgroup due to the inclusion of two studies only. Chang et al. [12] reported comparably high rates of en-bloc resection for both GA and sedation groups (95.7% vs. 97.9%, P = 0.68). Similar results were reported by Yurtlu et al. [17] as well (97.3% vs. 98.1%, P = 0.24). The study with mixed ESD indications did not report on en-bloc resection rates in the subgroups and was excluded from the subgroup analysis [18].
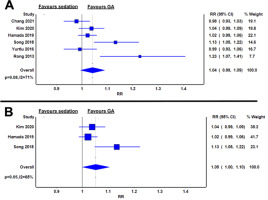
Procedural times
All seven studies reported on ESD procedural times, as defined by the time from initiation to termination of ESD [12–18]. The procedural times were comparable in GA and sedation (mean difference 3.47 minutes; 95% CI: –19.42 to 26.35; I2 = 97%; P = 0.77) (Figure 3A).
FIGURE 3
Weighted mean difference of procedural time between general anaesthesia and sedation groups in the whole cohort (A) and in oesophageal endoscopic submucosal dissection only (B)
When stratified by ESD subtype, procedural times were statistically comparable between GA and sedation in oesophageal ESD (mean difference 11.75 minutes; 95% CI: –9.03 to 32.53; I2 = 85%; P = 0.27) (Figure 3B) [13–16].
Only two studies reported procedural times in gastric ESD patients. Chang et al. [12] reported longer procedural times in GA patients than in sedation patients for gastric ESD (103.9 vs 82.4 minutes, P = 0.003). In contrast, Yurtlu et al. [17] reported a numerically shorter but statistically comparable procedural time in GA and in sedation (36.3 vs. 44.4 minutes, P = 0.094). The study with mixed ESD indications did not report procedural times in the subgroups and was excluded from the subgroup analysis [18].
Secondary outcomes
Intra-procedural vital instability
Intra-procedural vital instability was reported in four studies [13–15, 18]. As various definitions were used in the different studies, qualitative assessment without quantitative comparison was performed. Rong et al. [18] reported no cases of hypotension or respiratory depression in either the GA or sedation group (definition of hypotension and respiratory depression not provided). Yurtlu et al. [17] reported one case of desaturation (oxygen saturation < 90%) in their GA group versus 10 cases of desaturation in their sedation group. Kim et al. [14] reported no cases of vital instability in GA patients versus two cases of fast atrial fibrillation (heart rate not provided) and one case of desaturation (oxygen saturation < 90%) in their sedation group. Hamada et al. [13] reported no cases of vital instability in GA patients versus seven cases of hypotension (systolic pressure < 90 mm Hg), five cases of bradycardia (heart rate < 50 beats per minute), three cases of bradypnea (respiratory rate < 8 per minute) and two cases of hypoxia (oxygen saturatio n < 90%) in their sedation group.
Gastrointestinal perforation
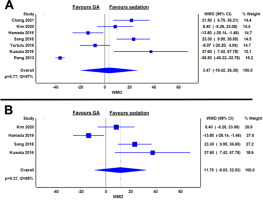
Six articles reported incidence of gastrointestinal perforation, defined by overt perforations seen on endoscopy or micro-perforations detected through post-procedural X-ray [12–17]. When compared with the sedation group, patients under GA had a relative risk of 0.62 to have gastrointestinal perforation, and this difference was borderline significant (RR 0.62; 95% CI: 0.21–1.82; I2 = 52%; P = 0.06) (Figure 4A).
FIGURE 4
Relative risks of gastrointestinal perforation between general anaesthesia and sedation groups in the whole cohort (A) and in oesophageal endoscopic submucosal dissection only (B)
Five studies reported on incidence of perforation in oesophageal ESD, and the relative risk of perforation was statistically comparable in GA and sedation (RR 0.41; 95% CI: 0.13–1.29; I2 = 23%; P = 0.27) (Figure 4B).
Only two studies reported perforation rates in gastric ESD, and both studies reported statistically comparable perforation rates between GA and sedation groups, respectively (3.3% vs. 5.6% and 12.8% vs. 5.3% respectively; both P > 0.05) [12, 18].
Gastrointestinal bleeding
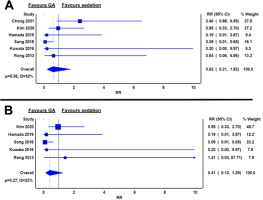
Six studies reported incidence of post-procedural gastrointestinal bleeding, defined as clinically overt gastrointestinal bleeding requiring further endoscopic therapy [12–16, 18]. No significant differences in bleeding risk were noted between GA and sedation (RR 0.75; 95% CI: 0.28–2.01; I2 = 0%; P = 0.48) (Figure 5A).
FIGURE 5
Relative risks of gastrointestinal bleeding between general anaesthesia and sedation groups in the whole cohort (A) and in oesophageal endoscopic submucosal dissection only (B)
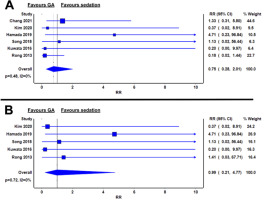
In oesophageal ESD, the relative risk of bleeding was similar between GA and sedation (RR 0.99; 95% CI: 0.21–4.77; I2 = 0%; P = 0.72) (Figure 5B).
For gastric ESD, two studies reported no significant difference in bleeding rates between GA and sedation groups, respectively (3.3% vs. 19.4% and 4.3% vs. 3.2%; both P > 0.05) [12, 18].
Aspiration pneumonia
Four studies reported on the incidence of post-procedural aspiration pneumonia, defined by post-procedural chest X-ray abnormalities [13–15, 17]. The risk of aspiration pneumonia was 0.24 in the GA group compared with sedation. However, this difference was not statistically significant (RR 0.24; 95% CI: 0.05–1.08; I2 = 0%; P = 0.75) (Figure 6A).
FIGURE 6
Relative risks of aspiration pneumonia between general anaesthesia and sedation groups in the whole cohort (A) and in oesophageal endoscopic submucosal dissection only (B)
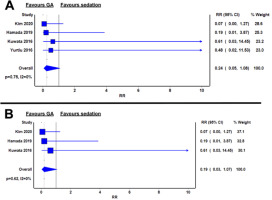
In oesophageal ESD, aspiration pneumonia rates were numerically lower in GA compared to sedation (RR 0.19; 95% CI: 0.03–1.07; I2 = 0%; P = 0.62) (Figu- re 6B).
For gastric ESD, Yurtlu et al. [17] reported aspiration pneumonia rates of 1.9% in their sedation group versus no cases in their GA group.
Risk of bias
Confounding
All the studies were cohort studies, and the assignment of patients to sedation or GA was dependent on the clinician’s discretion. This led to potential confounding by baseline tumour status and the presence of comorbidities. Three studies had a moderate risk of confounding [12, 14, 16], as the authors attempted to control for important confounders by regression or stratification. Four studies had a high risk of confounding [13, 15, 17, 18], as confounders were not addressed in the analysis.
Participant selection
Six studies had low bias risk in participant selection [12–16, 18]. One study had a high bias risk for participant selection, as the study rejected the first 40 patients from the analysis to account for the learning curve of ESD, yet the first 40 patients all received sedation [17].
Classification of intervention
All studies had low bias risk in the classification of intervention [12–18].
Deviations from intended interventions
All studies had a low risk of bias for deviation from intended interventions [12–18].
Outcome measurement
All studies had a low risk of bias in outcome measurement, as all outcomes were objective measures [12–18].
Selective reporting
The risk of bias in selective reporting was low in three studies [12, 13, 15]. Two studies had a moderate risk of bias, as multiple analyses and stratification of the same outcomes were noted [14, 16]. Two studies presented different outcomes or cut-offs for analysis in their methodology and result sections, leading to high bias risk in selective reporting [17, 18].
Overall risk of bias
The overall risk of bias was moderate in three studies [12, 14, 16] and high in four studies [13, 15, 17, 18]. Table 2 depicts the breakdown of the risk of bias.
TABLE 2
Risk of bias assessment
DISCUSSION
Our meta-analysis demonstrated that the en-bloc resection rate in oesophageal ESD was higher in GA when compared with sedation. En-bloc resection is associated with lower rates of tumour recurrence, which is a crucial advantage of ESD over other endoscopic resection methods [19, 20]. As the oesophagus has a thin wall and narrow lumen, a stable working field is required for successful en-bloc resection [16]. GA obviates the risk of undersedation and likely results in less inadvertent patient movement than sedation [8], which can explain its impact on increasing en-bloc resection rates in oesophageal ESD. The impact of GA on en-bloc resection was not observable for gastric ESD, and this may be explained by the larger lumen size of the stomach, which provides greater endoscopic manoeuvrability. As gastric ESD is less technically demanding than oesophageal ESD [21], the benefit of minimising patient movement through GA may be less apparent in gastric procedures.
The procedural times in our meta-analysis had high heterogeneity (I2 = 97%), and no significant difference in procedural time between GA and sedation was noted in the pooled analysis. Previous studies have reported tumour size and anatomical location as the key determinants of ESD procedural times [22, 23], as these factors can affect dissection speed. Other factors, including patient factors, have not been demonstrated to affect ESD procedural times [22, 23]. Future studies should aim to further characterise factors affecting procedural time, as endoscopic procedural time has potential implications for the efficiency of endoscopy suite operation [24]. Of note, the studies included in this review defined procedural time from initiation to completion of ESD but did not report on the time required for patient preparation and anaesthesia. The total patient contact time, including anaesthetic, pre-procedural preparation, and procedural and post-procedural recovery times should be reported in subsequent studies, as these data have important implications for endoscopy suite utility and cost-effectiveness.
Our meta-analysis also demonstrated a trend towards lower gastrointestinal perforation risk in GA when compared with sedation in ESD. As perforation is a rare event, the relatively small number of patients in this meta-analysis may mean the study is underpowered to investigate the association between GA and perforation. Gastrointestinal perforation is one of the most serious complications of ESD [20]. While perforations in ESD may be managed conservatively, cases requiring emergency surgery have been reported [25, 26]. Measures to prevent a perforation in ESD include adequate hands-on training, selection of suitable ESD equipment, and modification of ESD techniques based on tumour location and size [3]. GA may provide a more stable working field for ESD [8], which may, in turn, reduce the risk of gastrointestinal perforation.
Intra-procedural desaturation appeared to occur less frequently in patients undergoing GA than in sedation for ESD, although the heterogenous outcome reporting precluded quantitative comparison. Aspiration is a common adverse event in ESD [27, 28], and an advantage of GA is that endotracheal intubation is performed, and continuous airway protection is maintained throughout the procedure [29]. The lower rates of intra-procedural desaturation in GA patients may be attributable to better intra- procedural airway protection. Furthermore, we also observed a numerically lower rate of post-procedural aspiration pneumonia in GA patients, supporting the potential impact of GA on reducing respiratory complications in ESD.
The incidence of intra-procedural hypotension and arrhythmia was incompletely reported in included studies, limiting our ability to draw meaningful conclusions on the cardiovascular effects of GA in ESD. Nonetheless, effective sedative dosing and patient tolerability to endoscopy can vary substantially among patients, leading to difficulty maintaining a stable and adequate sedation level [29, 30]. Repeated dosing of sedatives during ESD may result in cardiovascular instability [29], and GA may be a more straightforward and safe option for maintaining intra-procedural cardiovascular stability.
Several limitations should be considered. First, the included studies all had relatively small sample sizes. The majority of included studies (six out of seven) were also retrospective studies. This meta-analysis may hence be underpowered to detect rare complications such as gastrointestinal bleeding or perforation. Multi-centre prospective studies or randomised trials should be considered for investigating the association between GA and ESD adverse events. Second, all studies had a moderate to high risk of bias, especially in confounding, which led to low evidence levels. The choice between GA versus sedation was based on clinician discretion in all the included studies, and potential confounding by baseline tumour status and patient comorbidities was possible. Indeed, two articles reported that GA patients had a larger total tumour size and circumferential tumour size when compared with the sedation groups in their respective studies [13, 16]. This suggested that clinicians may be more likely to perform GA in patients with anticipated difficult ESD. Given that more difficult cases were selected for GA, the direction of bias would likely favour the sedation group, and efficacy or safety outcomes would be biased against GA. Nonetheless, GA was associated with higher en-bloc resection rates in oesopha-geal procedures and trended towards lower rates of perforation, desaturation and post-procedural aspiration pneumonia despite potential bias. The results from this meta-analysis appear promising, and the efficacy and safety of GA in ESD warrant further investigation.
Another limitation of this meta-analysis is the limited number of gastric ESD studies [12, 17, 18], which limited subgroup analyses. Furthermore, no evidence of GA in colorectal ESD was identifiable on the systematic literature review. With the promising data on GA in upper gastrointestinal ESD, the use of GA in colorectal ESD should be explored as well.
CONCLUSIONS
The en-bloc resection rates in oesophageal ESD may be higher when using GA when compared with sedation. GA also demonstrated a trend towards lower rates of gastrointestinal perforation, intra-procedural desaturation and post-procedural aspiration pneumonia. Nonetheless, the risk of bias was moderate to high, and the level of evidence was low. High-quality, large-scale trials are warranted before the regular implementation of GA in ESD.