Introduction
An increased incidence of arrhythmia has been reported in pregnancy and it poses a therapeutic challenge [1, 2]. Incessant tachycardia in pregnancy can result in adverse maternal and fetal outcomes, including prematurity, intrauterine growth retardation, respiratory distress and congenital heart disease [3]. Persistent tachycardia may be asymptomatic but may develop maternal cardiomyopathy [4]. Antiarrhythmic drug therapy for maternal tachycardia is potentially harmful to the fetus, particularly in the first trimester, due to the potential risk of teratogenicity [4]. Radiofrequency (RF) catheter ablation is not encouraged during pregnancy, owing to the potential risks of catheter ablation in pregnant patients, such as radiation exposure and the possible hazard to the maternal and fetal health [5]. Thus, catheter ablation has been avoided during pregnancy because of the requirement for fluoroscopy [6], and a postponed RF to the postpartum period was always recommended [7].
Conventional RF catheter ablation using minimal fluoroscopy determines the cardiac anatomy and navigates the catheter for terminating arrhythmias, while minimizing ablation-associated ionizing radiation and complications [2]. However, such reports were usually limited to supraventricular tachycardia (SVT) treatment. Ablation of ventricular tachycardia (VT) in patients with structural heart disease was often a longer procedure under fluoroscopy and general anesthesia. It was reported that VT ablation was successfully performed in a pregnant patient with arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) at gestation week 36 [8]. Moreover, when the procedure was done in an awake patient and the mechanism of tachycardia was atrioventricular nodal, cryoenergy was preferred over radiofrequency [1]. Catheter ablation of tachycardias may be safely and successfully performed with minimal radiation exposure in pregnancy with the guidance of three-dimensional (3D) mapping systems. With the advent of 3D mapping systems, the need for fluoroscopy during catheter ablation has been substantially reduced [7]. 3D mapping facilitates the recreation of the geometry of the concerned cardiac chambers by tracing the mapping catheter around the chamber [2]. However, there is a concern of fetal exposure to radiation, which may result in fetal abnormalities including intellectual disability, in particular, with a dose of > 50 mGy [9, 10]. Nowadays, catheter ablation for tachyarrhythmias during pregnancy under minimal radiation or zero-fluoroscopy is continuously reported. However, the management and the overall outcome of this condition are still unknown.
Aim
The aim of the present study is to give an overview of catheter ablation for tachyarrhythmias during pregnancy, and to discuss the indications of the procedure and the outcomes of both mother and fetus.
Material and methods
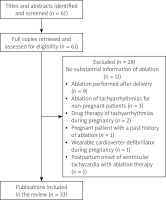
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement guidelines were followed in this review. Comprehensive retrieval from PubMed, Google Scholar and “Baidu” Scholar was conducted for articles published from 2000 to 2022. The search terms included “arrhythmia”, “tachyarrhythmia”, “tachycardia”, “supraventricular tachycardia”, “atrial fibrillation”, “atrial flutter”, “ventricular fibrillation”, “premature atrial contraction”, “premature ventricular contraction”, “atrioventricular nodal reentrant/reciprocating tachycardia”, “permanent junctional reciprocating tachycardia”, “Wolf-Parkinson-White syndrome”, “accessory pathway”, “ablation”, “radiofrequency”, “cryo-ablation”, “fluoroscopy”, “zero-fluoroscopy”, “pregnancy”, “parturient” and “peripartum”. The inclusion criteria were prospective or retrospective studies, case series, case reports and proceeding abstracts of ablation of tachyarrhythmia during pregnancy. The primary exclusion criteria were publications: with no substantial information of ablation (n = 11), ablation performed after delivery (n = 9), ablation of tachyarrhythmias for non-pregnant patients (n = 3), drug therapy of tachyarrhythmias during pregnancy (n = 2), pregnant patient with a past history of ablation (n = 1), wearable cardioverter-defibrillator implant during pregnancy (n = 1), and postpartum onset of VT receiving ablation therapy (n = 1). Moreover, 1 patient who received ablation after abortion was excluded from an included report [36]. As a result, a total of 33 articles were included and 28 articles were excluded. A flowchart of literature inclusion is shown in Figure 1.
Statistical analysis
IBM SPSS statistics version 22 software was applied for statistical analysis. The measurement data were expressed as mean ± standard deviation and were compared by independent or paired t test, while the categorical data were expressed as numbers and percentages and were compared by χ2 or Fisher exact test with continuity correction. P < 0.05 was considered of statistical significance.
Results
A total of 33 articles were included including 128 patients with tachyarrhythmias indicated for an ablation [1, 2, 4, 7, 8, 11–37]. One patient had a recurrent SVT during pregnancy after the initial ablation and a reintervention was warranted in the second trimester [18], and another patient had a failed indwelling jugular vein catheter during anesthesia for ablation and was thus withdrawn from the ablation list [23]. Therefore, there were 129 occasions in 128 patients for partly preprocedural and 128 occasions in 127 patients for all postprocedural evaluations. The articles were 23 (69.7%) case reports [1, 2, 4, 7, 8, 15–22, 24, 26–29, 31–35], 6 (18.2%) case series [11, 13, 14, 30, 36, 37], 3 (9.1%) retrospective research articles [3, 23, 25] and 1 (3.0%) proceeding abstract [12].
Patients were at the age of 29.8 ±6.0 (range: 18–48; median: 29.8) years (n = 48). On current admission due to tachyarrhythmias, they were at 22.8 ±8.0 (range: 6–38; median: 24) weeks of gestation (n = 36). Three (2.3%) patients had twin pregnancies [1, 4, 11]. Three (2.3%) were pregnant by in vitro fertilization [4, 18, 26], and one of them had a twin pregnancy [4]. Their parity number was 2.0 ±1.7 (range: 1–9; median: 1) [2, 3, 8, 11, 14, 16, 17, 23, 24, 27, 28, 30–32, 36].
Tachyarrhythmias occurred before pregnancy in 24 (42.9%) patients [3, 7, 8, 11, 13, 21, 25, 27–29, 31, 36] and they occurred during pregnancy in 32 (57.1%) patients [2, 3, 14, 16, 17, 19, 24–26, 30, 33, 35, 37], whereas the onset time was unspecified for 72 patients. No difference was found in the prevalence of tachyarrhythmic onset between before and during pregnancy (χ2 = 2.286, p = 0.093). Of the 32 patients with a new-onset arrhythmia during pregnancy, the gestational age of 16 patients was known. Arrhythmias occurred in 3 (18.8%), 9 (56.3%) and 4 (25%) patients in the three trimesters, respectively (χ2 = 5.8, p = 0.055). Among patients with an onset of tachyarrhythmias before pregnancy, the preexisting arrhythmias exacerbated after pregnancy in 7 (29.2%, 7/24) patients [7, 8, 11, 13, 31, 36]. A previous history of ablation was recorded for 4 (3.1%, 4/128) patients [3, 8, 21, 29].
The clinical presentations were described for 34 patients. One of them was asymptomatic [4], and 33 patients had a total of 60 clinical presentations, including palpitations in 24 (40%) [1–3, 7, 11, 13, 15, 17, 19–21, 26–28, 30, 31, 33, 35, 36], dyspnea in 6 (10%) [2, 13, 16, 19, 26, 37], and breathlessness/shortness of breath in 5 (8.3%) [26, 31, 35, 37]. Patients’ heart rate at presentation was 202.3 ±43.7 (range: 150–300; median: 187) beats/min (n = 31) [1–4, 8, 11, 13–16, 18–20, 22, 26, 28, 30, 31, 33, 35, 37]. The left ventricular ejection fraction was 44.9 ±16.0 (range: 18–70; median: 41)% (n = 25) at presentation.
The indications for ablation were not given for 55 (43.0%) patients [3, 12, 23, 32, 37], while detailed indications were known for the remaining 73 (57.0%) patients. Unresponsiveness of arrhythmia to drug therapy was an indication for an ablation therapy in most patients (Table I). In 32 patients, 64 antiarrhythmic drugs showed poor or no effect on arrhythmias in pregnant women. Of these drugs, β-blockers (including metoprolol, sotalol, propranolol, labetalol and esmolol) were the most commonly used, representing 48.4% (31/64), followed by calcium antagonists (verapamil and diltiazem), representing 15.6% (10/64). Types of arrhythmias during pregnancy are shown in Table II, with atrioventricular nodal reentrant tachycardia being the most common.
Table I
Indications for ablation therapy
| Indication | n (%) |
|---|---|
| Unresponsive to drug therapy [2, 3, 7, 8, 11, 13, 14, 17, 18, 20, 21, 23–26, 28–31, 33–37] | 59 (80.8) |
| Requiring electrical cardioversion [3, 13, 19, 23] | 5 (6.8) |
| Unresponsive to drug therapy + failed electrical cardioversion [3, 16, 22] | 3 (4.1) |
| Unresponsive to drug therapy + requiring electrical cardioversion [3] | 2 (2.7) |
| Unresponsive to drug therapy + vagal maneuver requirement [1] | 1 (1.4) |
| Refusal of drug therapy [15] | 1 (1.4) |
| For ventricular fibrillation [27] | 1 (1.4) |
| Anticipated further deterioration in left ventricular function [4] | 1 (1.4) |
Table II
Types of tachyarrhythmias during pregnancy
| Arrhythmia | n (%) |
|---|---|
| AVNRT [1, 3, 12, 14, 15, 23–25, 28, 30] | 32 (24.8) |
| AT [2–4, 7, 13, 16, 23, 25, 26, 29, 31, 37] | 21 (16.3) |
| PVC [11, 23, 25] | 13 (10.1) |
| PVC + VT [8, 12, 17] | 10 (7.8) |
| SVT [14, 14, 18, 20, 30, 33, 35] | 8 (6.2) |
| WPW [21, 23, 25] | 7 (5.4) |
| PJRT [3, 23] | 4 (3.1) |
| AF [13, 25] | 3 (2.3) |
| AVRT [19, 30, 32] | 3 (2.3) |
| VT [25] | 3 (2.3) |
| WPW-AF [3] | 2 (1.6) |
| Atrial flutter [34] | 1 (0.8) |
| AVRT + PVC [36] | 1 (0.8) |
| AVRT-AF-VF-WPW [27] | 1 (0.8) |
| WPW-AVRT [3, 11] | 1 (0.8) |
| SVT + PAC [22] | 1 (0.8) |
| Others | 17 (13.2) |
[i] AF – atrial fibrillation, AT – atrial tachycardia, AVNRT – atrioventricular nodal reentrant tachycardia, AVRT – atrioventricular reciprocating tachycardia, PJRT – permanent junctional reciprocating tachycardia, PVC – premature ventricular contraction, SVT – supraventricular tachycardia, VF – ventricular fibrillation, VT – ventricular tachycardia, WPW – Wolff-Parkinson-White syndrome.
57 arrhythmic sources of 53 patients were reported, with the right ventricular outflow tract and the tricuspid annulus being the most common arrhythmic sources (Table III).
Table III
The 57 arrhythmic sources of 53 patients
| Arrhythmic source | n (%) |
|---|---|
| Right ventricular outflow tract [8, 11, 17, 25] | 9 (15.8) |
| Tricuspid annulus [11, 18, 19, 25, 28, 30, 35] | 7 (12.3) |
| Left atrial appendage [25, 26] | 5 (8.8) |
| Right septum [1, 7, 14, 36] | 5 (8.8) |
| Mitral annulus [13, 14, 30, 33] | 4 (7.0) |
| Right atrial appendage [2, 13, 25] | 4 (7.0) |
| Coronary cusp [25] | 3 (5.3) |
| Crista terminalis [4, 22, 31] | 3 (5.3) |
| Left atrial wall [16, 29, 32] | 3 (5.3) |
| His bundle/His cloud [24, 28] | 2 (3.5) |
| Fascicular [25] | 2 (3.5) |
| Para-Hisian [7, 21] | 2 (3.5) |
| Cavo-tricuspid isthmus [34] | 1 (1.8) |
| Coronary sinus [28] | 1 (1.8) |
| Great cardiac vein [36] | 1 (1.8) |
| Koch’s triangle [15] | 1 (1.8) |
| Mitral isthmus [20] | 1 (1.8) |
| Papillary muscle [25] | 1 (1.8) |
| Right atrium wall [37] | 1 (1.8) |
| Sinus venosus [37] | 1 (1.8) |
An accessory pathway was identified in 28 patients: a left accessory pathway in 16 (57.1%) [3, 12–14, 20, 24, 29, 30, 32], a right accessory pathway in 10 (35.7%) [11, 12, 19, 21, 27, 30], a posteroseptal accessory pathway in 1 (3.6%) [12] and a Coumel accessory pathway in 1 (3.6%) patient [14].
Tachycardiomyopathy was present in 6 (4.7%) patients [4, 13, 31, 34, 37], and one of them was associated with dilated cardiomyopathy [37]. One (0.8%) patient had ARVD/C [8]. The remaining 121 (94.5%) patients had a structurally normal heart.
The ablation time was recorded for 47 patients. It was performed at 23.5 ±7.8 (range: 10–38; median: 24) weeks of gestation: in the first trimester in 7 (14.9%) patients [3, 7, 13, 15, 22, 26, 30], in the second trimester in 23 (48.9%) patients [1–3, 8, 11, 16, 19–21, 23–25, 27, 29–32, 34, 35, 37] and in the third trimester in 17 (36.2%) patients [3, 4, 11, 13, 14, 17, 18, 23, 28, 30, 33] (χ2 = 12.5, p = 0.002). One of the patients had ablations twice in the third trimester due to recurrence of arrhythmia after the first ablation [18]. Ablation was performed under fluoroscopy in 21 (16.4%) patients [2, 3, 8, 13, 14, 17, 18, 20, 31, 33, 36, 37], and zero-fluoroscopy technique was applied in 108 ablations of 107 (83.6%) patients [1, 3, 4, 7, 11, 12, 15, 16, 19, 21–30, 32, 34, 35] (χ2 = 115.6, p < 0.001). The fluoroscopic exposure lasted 322.8 ±439.1 (range: 55–1,776; median: 109) s (n = 15), and the radiation dose was reported for 16 patients, with a mean of 9.6 ±10.3 (range: 0.1–35.1; median: 10) mGy (n = 10). The radiation dose was within 50 mGy in 11 (68.8%) patients [2, 3, 8, 13, 17, 18, 33, 37] and > 50 mGy in 5 (31.2%) patients [3] (χ2 = 4.5, p = 0.034).
The guiding system was recorded for 120 patients including the CARTO 3D system in 64 (53.3%) patients [12, 19, 22, 23, 25, 26, 28, 29, 33, 35], the Ensite NavX system in 44 (36.7%) patients [1, 2, 4, 7, 11, 12, 16–18, 21, 23, 24, 27, 30, 32, 34, 37], an electroanatomical system in 7 (5.8%) patients [3, 8, 36], fluoroscopy guidance in 4 (3.3%) patients [3] and intracardiac echocardiography in 1 (0.8%) patient [15].
The circle length was reported for 11 patients with a mean of 383.9 ±153.4 (range: 220–695; median: 310) ms [4, 16, 20, 21, 24, 26, 28, 29, 31, 35, 37]. There were 67 accesses reported for 56 patients, including right femoral vein in 16 (23.8%) [1, 2, 4, 7, 11, 14, 17, 19, 21, 26, 33, 35–37], right internal jugular vein in 10 (14.9%) (it failed in one of the patients) [23], femoral veins or arteries (indistinct description) in 8 (11.9%) [3], right femoral artery in 4 (6.0%) [7, 14, 21, 36], femoral artery (laterality unspecified) in 3 (4.5%) [8, 16, 23], left femoral vein in 3 (4.5%) [15, 33, 34], left subclavian vein in 3 (4.5%) [14, 16], femoral vein (laterality unspecified) in 2 (3.0%) [16, 24], and transseptal in 18 (26.9%) patients [16, 20, 21, 25, 26, 29, 30, 32] (via the patent fossa ovalis in one of them [30]).
In 127 patients, 128 ablations were performed including 126 (98.4%) RF and 2 (1.6%) cryo-ablations [1, 23]. Moreover, 1 patient had concurrent left superior vein isolation [29], and another patient received an emergent RF ablation [27]. The RF energy applied for ablations was 39.0 ±13.8 (range: 20–60; median: 35) W (n = 15) [2, 7, 14, 16, 19, 22, 26, 28, 33, 35, 36]. The RF temperature was 58.6 ±3.8 (range: 55–65; median: 60) °C (n = 7) [7, 14, 16, 35, 36]. The duration of ablation was 80.1 ±99.4 (range: 2–320; median: 60) s (n = 15).
The application number of RF or cryo-ablations was described for 32 patients. In 1 patient, it was described as “several” [18], and the number of applications in the remaining 31 patients was 119 in total with 95 (79.8%) successful [1–4, 8, 11, 13, 15, 17, 19, 21, 24, 26, 29, 30, 31, 33–37], and 24 (20.2%) failed applications [2, 4, 11, 19, 21, 30, 35] (χ2 = 84.7, p < 0.001). The duration of the procedures was 76.6 ±40.5 (range: 29–186; median: 71) min (n = 26) [1–3, 7, 11, 12, 14, 15, 21, 24–26, 29, 30]. In 45 patients, an immediate arrhythmic termination after ablation was reported [4, 7, 8, 17, 18, 22, 25, 27–34, 36, 37]. No programmed extrastimuli-induced tachycardia was reported for 20 patients [7, 8, 14, 15, 18, 19, 21, 24, 26, 28–31, 33, 35]. No recurrence of arrhythmia during 30-min observation after the procedure was reported for 6 patients [7, 15, 17, 18, 22, 26]. There were 2 (1.6%) procedural complications: iliofemoral thrombosis in one [12] and severe hypoxemia, tachypnea and hypotension in another among 128 procedures [37]. Pregnant complications were present in 3 (2.4%, 3/127) patients including placental abruption [12], preeclampsia and placenta separation [3] and a spontaneous abortion [37] in 1 patient, each. There was no mortality for the pregnant patients. The left ventricular ejection fraction was 47.1 ±12.6 (range: 25–68; median: 50)% (n = 8). In patients with both pre- and postprocedural ejection fraction results, a paired t test revealed that these patients had a significantly higher postprocedural ejection fraction than before (preprocedural 30.7 ±10.3% vs. postprocedural 46.3 ±13.4%, t = –3.516, p = 0.013).
Three women had twin pregnancies, and thus there were a total of 129 fetuses for 127 pregnant patients. In 4 (3.1%, 4/127) cases, there was an adverse event/complication including amniocentesis [14], retardation in cranial growth [14], induced abortion soon after the ablation due to the expectation that the early interruption of the pregnancy might prevent any progression of her cardiomyopathy [36] and a spontaneous abortion [37]. The mean Apgar scores of the newborns were 9.0 ±1.0 (range; 8–10; median; 9) (n = 5) [8, 14, 25], 9.5 ±0.6 (range: 8.8–10; median: 9.9) (n = 5) [12, 14, 25] and 10 (n = 1) [25] at 1, 5 and 10 min, respectively. The incidence of fetal adverse events was higher in the fluoroscopy than in the zero-fluoroscopy group [14.3% (3/21) vs. 0.9% (1/107), χ2 = 9.9, p = 0.015].
In 1 case report [36], the gestational age was not mentioned, and in another report with 11 cases in which the patient was withdrawn from the ablation list due to a failed indwelling catheter, the gestational ages were not reported clearly [23]. Thus, trimester grouping included 7 (5.5%), 104 (81.3%) and 17 (13.3%) ablations in the three trimester groups, respectively (the case of reintervention was included in the second trimester group). All three fetal adverse events occurred in the second trimester, and the incidence of fetal adverse events of the three groups was 0% (0/7), 2.9% (3/104) and 0% (0/17), respectively (χ2 = 0.7, p = 0.702).
The gestational age at normal delivery was 37.4 ±2.2 (range: 33–41; median; 37) weeks (n = 17) [1, 4, 7, 8, 11, 12, 14, 17, 21, 25, 31, 34, 35]. Irrespective of the 2 cases with an early abortion, the delivery mode was reported for 61 (47.7%) patients: a cesarean section in 20 (32.8%) [1, 3, 4, 7, 8, 11, 12, 14, 17, 21, 26, 31, 33] (in 3 of them, the indications for cesarean section were described as maternal idiopathic chronic intestinal subocclusion, failure to progress and fetal distress, respectively [14]), a vaginal delivery in 41 (67.2%) patients (spontaneous vaginal delivery in 37 [12, 28, 35] and vaginal-assisted delivery in 4 patients [12]). The patients were on a follow-up of 12.3 ±11.8 (range: 1–43; median: 6) months (n = 24) [1, 3, 4, 8, 11, 12, 14, 16–21, 25, 31–34, 36, 37]. Of the 128 procedures in 127 patients, the outcomes were fine in 125 (97.7%) ablations of 124 patients and arrhythmic recurrence occurred in 3 (2.3%) ablations of 3 patients. The 3 recurrences were self-cured in 2 [26, 31] and a reintervention was required during the pregnancy in 1 patient [18]. The overall reintervention rate was 0.8% (1/128).
Discussion
Mechanisms
Most arrhythmias in pregnant women are benign and severe arrhythmias requiring aggressive therapies are rare [38]. Pregnancy may trigger an exacerbation of preexisting arrhythmias, whereas other arrhythmias may occur for the first time. Tawam et al. [39] found an increased risk of both new-onset (34%) and exacerbation (29%) of SVT in pregnancy. Jastrzębski et al. [18] stated that, in pregnant patients with SVT before pregnancy, about 22–44% were exacerbations of a preexisting arrhythmia, whereas the arrhythmia was a new-onset in 3.9% of the cases. In pregnant patients with a previous arrhythmia, recurrence and exacerbation during pregnancy are common and often exert an unfavorable effect on fetal and neonatal outcomes [40]. The maternal arrhythmias are a more frequent recurrence of a previously diagnosed arrhythmia in patients with structural heart disease [41]. This study revealed an exacerbation of a previous arrhythmia in 29.2%, very close to what the above-cited Tawam et al. [39] reported, whereas the new onset incidence was 57.1%. The exact mechanism of tachyarrhythmias during pregnancy was uncertain, but the hyperdynamic state of pregnancy was considered the major contributing factor. Pregnancy per se is a predictive risk of arrhythmias in pregnant patients with normal or abnormal heart structures [17]. The predisposing factors responsible for the development or exacerbation of arrhythmias during pregnancy include remarkable hemodynamic changes, fluctuations in hormone levels throughout the body, autonomic tone changes, enhanced sensitivity to circulating catecholamines, hypokalemia during pregnancy and presence of organic cardiac disease [17].
Pregnancy has been identified as a risk factor for paroxysmal SVT. An expanded circulating volume may increase myocardial irritability and a faster sinus heart rate may alter tissue excitability, initiating a reentry circuit. Estrogens may increase cardiac excitability just as they affect uterine muscle, and thus the myocardium becomes sensitive to catecholamines, with a dramatic increase in the number of α-receptors. Moreover, peripartum oxytocic, tocolytic and anesthetic drugs have also been suggested as triggers for inducing SVT [42].
An increased sensitivity to circulating catecholamines during pregnancy has also been proposed as a potential trigger for VT [43]. The rate of new-onset VT during pregnancy in patients with organic cardiac disease was much higher than in those without, and the new-onset VT showed an equal distribution among the three trimesters [17]. Circulating estrogen has been doubted as a mechanism of VT initiation in pregnancy [17]. The antiarrhythmic effect of estrogen has been considered a result of its calcium antagonistic properties in cardiac myocytes, or an imbalance between dominant progesterone and estrogen [17]. The VTs developing in pregnant patients with no organic cardiac disease were reported to be the monomorphic type and responded well to β-blocker therapy [44]. Atrial fibrillation and atrial flutter rarely occur during pregnancy, mostly secondary to congenital or valvular heart disease, or metabolic disturbances including thyrotoxicosis and electrolyte disturbance [44].
Guidelines
Treatment of arrhythmias in pregnancy poses challenges as for the concern of potential fetal adverse effects. The American Heart Association (AHA)/American College of Cardiology (ACC)/European Society of Cardiology (ESC) published their guidelines for the management of patients with supraventricular arrhythmias in 2003 [45], in which the treatment strategies for SVT during pregnancy were proposed as follows: 1) acute conversion: vagal maneuver, adenosine, electrical cardioversion, metoprolol, propranolol and verapamil; and 2) prophylactic therapy: digoxin, metoprolol, propranolol, sotalol, flecainide, quinidine, propafenone, verapamil, procainamide, catheter ablation, atenolol and amiodarone. Afterwards, the European Society of Cardiology (ESC) launched its first and second ESC guidelines on the management of cardiovascular diseases during pregnancy in 2011 and 2018, respectively [46, 47]. In the above guidelines, the acute management strategies for SVT included vagal maneuver, electrical cardioversion, β-blockers and verapamil, whereas β-blockers, propafenone, procainamide, verapamil, etc., were reserved for long-term management. In the first ESC guideline, ibutilide or flecainide was proposed for termination of atrial flutter and atrial fibrillation in stable patients with structurally normal hearts, and atenolol was recommended not to be used for any arrhythmia. The recommendations in the 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia [48] and the 2019 ESC Guidelines for the management of patients with supraventricular tachycardia [49] were similar to those in the previous guidelines except for intravenous and oral amiodarone included for acute and ongoing management strategies respectively in the former [48], and catheter ablation recommended in symptomatic women with recurrent SVT planning to be pregnant and avoidance of all antiarrhythmic drugs during the first trimester in the latter [49]. Catheter ablation was Class II at Level C of the long-term management strategy in all guidelines and it was recommended to be performed in the second trimester.
According to the above guidelines, antiarrhythmic drugs should be avoided in the first trimester if possible. In hemodynamically stable pregnant patients with SVT, a vagal maneuver should be tried first, and electrical cardioversion is essential. If they fail, intravenous adenosine as well as digoxin, β-blockers, or calcium-channel antagonists should be considered. The use of β-blockers is usually considered safer, especially in late pregnancy, and calcium-channel antagonists are also considered safe in the second and third trimesters for terminating the arrhythmias. In stable patients with VT, lidocaine and procainamide can be used. Sotalol can be a choice when other β-blockers are ineffective [44]. The guidelines suggested that catheter ablation should be considered in pregnant patients with drug-refractory and poorly tolerated tachycardias [50].
Radiation exposure
With the widespread use of electroanatomic mapping and intracardiac echocardiography, future radiation risks may be reduced, or fluoroscopy will not be required at all [5]. Abdominal shielding should be routinely used in pregnancy [5]. Radiation exposure to the fetus should be minimized particularly in early pregnancy due to the concern of organogenesis and neuronal development. Embryos in the earliest stages are most susceptible to radiation, and an exposure of 100 mGy in an embryo at 2-week gestation may cause embryonic death. It was known that the threshold dose for fetal abnormalities was 100–250 mGy in a gestation of < 8 weeks, and it was 60–310 mGy in 8–25-week gestation. Antenatal radiation exposure of 10 mGy may increase the risk of childhood cancer [51], and a radiation exposure of much less than 50 mGy can be a threshold value for noncancerous health effects in the case of ionizing radiation [52]. Damilakis et al. [53] found that fetal radiation exposure < 1 mGy with abdominal shielding during catheter ablation for refractory arrhythmias in pregnant patients was associated with good maternal and fetal outcomes. In general, a fetal radiation dose of < 1 mGy can be a safe threshold for pregnant patients undergoing SVT ablation [54].
Catheter ablation for arrhythmias has been successfully performed in pregnant patients with low radiation exposure and has achieved good outcomes [3]. Zero-fluoroscopy, i.e., catheter ablation by using electroanatomic mapping systems and intracardiac echocardiography to reduce fluoroscopic exposure, has been safely performed during pregnancy for drug-refractory and poorly tolerated arrhythmias. Li et al. [25] reported that, for patients with SVT and VT, three multipolar diagnostic catheters were inserted into the coronary sinus, His bundle region and right ventricular apex through the femoral veins. Intracardiac echocardiography-guided zero-fluoroscopy transseptal puncture technique was applied for left-sided arrhythmic ablations. Chen et al. [11] summarized information of 12 patients undergoing catheter ablation during pregnancy from 8 published articles. The tachyarrhythmias were atrial tachycardia in 3, atrioventricular nodal reentry tachycardia in 3, atrioventricular reciprocating tachycardia in 4 and persistent junctional reciprocating tachycardia in 2 patients. All RF ablations were successfully performed and both women and fetuses had an uneventful postprocedural course with no recurrence or complications.
Challenging aspects
For these rare clinical situations, some challenging aspects in relation to catheter ablation during pregnancy including drug challenges (isoproterenol) or the use vascular access, gestational age, anesthesiologist presence during the procedure and the guidelines or practical clinical management strategies for peri-procedural assessment of fetus status and implementation of this approaches in different countries and health care systems should be addressed (Table IV) [55–61]. Isoproterenol is a β-receptor agonist for bronchial asthma and atrioventricular block, but it may also reduce β1-mediated tachycardia [56]. Vascular access of choices is based on the principle of convenient maneuvers, and the right femoral vein is the preferable access in most cases. Local anesthesia is usually enough for the ablation procedure [1, 7]; however, the presence of anesthesiologists is important for safety for drug use during ablation avoiding preterm labor and delivery. Even though the guidelines are practiced in different countries, the recommendations are largely identical but with minor differences. If possible, drugs, electrophysiological study and interventional diagnosis and therapy should be avoided in the first trimester due to the possible harmful consequences for the fetuses [48].
Table IV
Interpretation of important aspects in relation to catheter ablation during pregnancy
| Important aspect | Interpretation |
|---|---|
| Drug challenges (isoproterenol) |
|
| Vascular access |
|
| Gestational age |
|
| Anesthesiologist presence during procedure |
|
| Guidelines or practical clinical management strategies in different countries and health care systems |
|
| Peri-procedural assessment of fetus and implementation of the approaches |
|
Multidisciplinary team
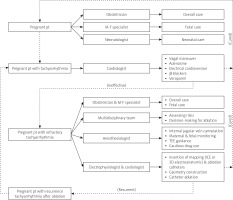
As pre-excitation is a predisposing factor for syncope recurrences and carries a potential risk to the fetus and mother [21], the care of pregnant patients with refractory arrhythmia is best done jointly by a multidisciplinary team including an obstetrician, a cardiologist, an electrophysiologist (or a cardiologist specializing in electrophysiology), a pediatrician, a neonatologist and an anesthesiologist [27, 33]. Careful assessment of the pregnant patient and fetus is mandatory. Sufficient preparation with a complete understanding of both cardiac anatomy and physiology and multidisciplinary consultation reaching the consensus of management strategies is a prerequisite of success of interventional therapy [62]. The pregnancy heart team, i.e., the cardio-obstetrics team, aims to provide specialized multidisciplinary care in the antepartum, peripartum, and postpartum periods to reduce risk by following a collaborative care plan to manage the high-risk cardiovascular patient during pregnancy, delivery and the postpartum period [63]. Cardiologists would be in charge of management of hypertension, medication safety used during pregnancy and lactation, maternal cardiovascular benefits of breastfeeding, long-term outcomes of pregnant patients, interpretation of biomarkers and echocardiography, hemodynamic changes during and after pregnancy as well as delivery. Cardiovascular indications for cesarean delivery require pertinent knowledge to avoid unnecessary surgical intervention [64]. Moreover, the obstetricians are in charge of the pregnant woman’s overall care, the maternal-fetal medicine specialists direct the care of the pregnant woman, neonatologists manage the care of neonates, and nurses, pediatric and surgical specialists, genetic counselors, chaplains, ethicists and members of institutional ethic committees are also included in the team [65]. The protocol of a multidisciplinary team in managing tachyarrhythmias in a pregnant patient is shown in Figure 2.
Interpretations of results
This review revealed that the indications for ablation were refractory arrhythmias unresponsive to drug therapy in most of the cases, and requiring cardioversion was another indication. The antiarrhythmic drugs that showed poor or no response to arrhythmias in pregnant women were β-blockers in most situations. Atrioventricular nodal reentrant tachycardia was the most common arrhythmia that developed during pregnancy. The onset of all tachyarrhythmias was not equal among the three trimesters. The arrhythmic sources were located in the right ventricular outflow tract and the tricuspid annulus in most cases. Fetal adverse events occurred more in fluoroscopy than in zero-fluoroscopy, even though the radiation dose was within the safe threshold in most of the cases. A radiation dose of > 50 mGy in one-third of the cases as shown in this report was associated with a 14.3% fetal adverse event rate. This supported the necessity of zero-fluoroscopy or strict minimal fluoroscopy. This review also revealed that fetal adverse events occurred only in the second trimester and not in the other two trimesters. This might be explained by a higher incidence of new-onset arrhythmias in the second trimester during pregnancy. Afanasiuk et al. [66] also observed a higher incidence of new-onset arrhythmias in the second trimester. They explained this trend as a result of compression of the cardiovascular structures by the enlarged uterus in the second trimester. For an ablation procedure, the patient should be placed in the left lateral tilt position to prevent aortocaval compression, especially after the second trimester of pregnancy [54].
In a word, catheter ablation should be considered in pregnant patients with drug-refractory and poorly tolerated tachycardias, particularly when the advantages of catheter ablation overweigh the disadvantages. However, catheter ablation may bring about potential risks to the mother and the fetus from fetal radiation exposure and fetal compromise due to maternal hemodynamic instability for the latter. Moreover, the gravid uterus may cause difficult patient positioning during the procedure.
Limitations
The study materials of this review were composed of mostly case reports and case series. Patient information was incomplete and missing in many reports. There was a lack of randomized trials on this topic. The usual follow-up of catheter ablation during pregnancy is provided until delivery. However, a very long term follow-up including pregnancy, peripartum, and breast-feeding has not been reported. These shortcomings constituted the main drawbacks of this study. Nonetheless, this report still offers some preliminary results for clinical reference.
Conclusions
Drug-refractory and poorly tolerated tachycardias in pregnant patients warrant catheter ablation. Zero-fluoroscopy technique under guidance with 3D mapping systems is preferred due to concern over radiation damage to the fetus. Strict minimal fluoroscopy is only used in extreme necessity during the ablation for pregnant patients. As ablation in the first and third trimesters may be associated with fewer fetal adverse events than in the second trimester, it is suggested that ablation is preferably performed in the third trimester. Effective collaboration of a multidisciplinary team is a prerequisite for success of catheter ablation therapy of maternal tachyarrhythmias.